Photo: Drexel University
Little Supercapacitors: Carbon particles store charge in a flow capacitor.
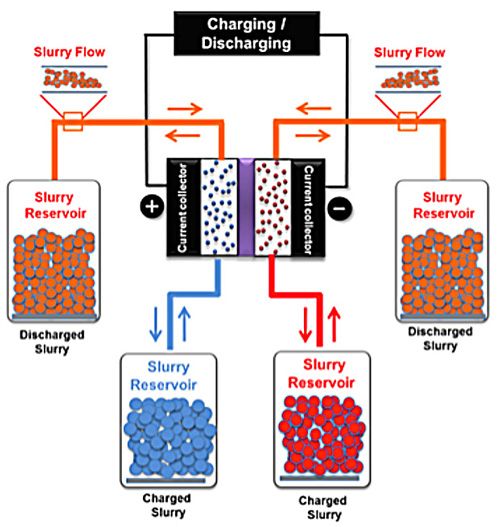
Illustration: Drexel University
Reversible Reservoirs: A carbon-particle slurry flows from one
reservoir to the other. It can pick up charge as it passes through an
electrochemical cell. Or give it up when flowing in reverse. Click on the image to enlarge.
A number of technologies—like flywheels, sodium sulfur batteries, and compressed-air storage—will eventually contribute to that storage, but these all have drawbacks: Some don’t store enough energy, some discharge it too slowly, and some lose their storage abilities after a few years of use. But new research out of Drexel University, in Philadelphia, suggests a technique that combines some of the best aspects of two disparate ideas—flow batteries and supercapacitors—that could hit the sweet spot of scalable and affordable energy storage.
The Drexel device, called an electrochemical flow capacitor (EFC), consists of an electrochemical cell—two electrode compartments separated by a membrane—that’s connected to two pairs of external reservoirs. Each reservoir contains a slurry of highly porous carbon particles suspended in a liquid electrolyte medium. When charging the system, the electrodes are polarized and slurry flows past them, gathering a double layer of charge on its way to the reservoirs. “Inside the cell, energy is stored capacitively at the surface of the carbon particles,” similar to what you’d see in a supercapacitor, says Emin C. Kumbur, who led the research at Drexel along with Yury Gogotsi.
The charged slurry can be stored in the reservoirs until it is needed, when it is pumped back through the cell and discharged. A similar flow concept is used in flow batteries, which store energy in a chemical reaction as electrolytes flow past. “Operationally, the system behaves much like a redox flow battery,” Kumbur says. “Fundamentally, however, the energy is stored in the same way as conventional supercapacitors.”
The primary advantage of the carbon-based slurry supercapacitor approach is that it does not wear out as quickly as other energy-storage technologies. Batteries—be they lead-acid, lithium-ion, or redox flow—are limited by the fact that the chemical reactions eventually degrade the battery’s chemicals and materials. Supercapacitors have much longer lifetimes without any such chemical reactions and degradation. According to the paper in Advanced Energy Materials by Kumbur and his colleagues, the EFC would, like a supercapacitor, be expected to last on the order of 100 000 or more cycles. Redox flow batteries and lithium-ion batteries, meanwhile, might last less than 15 000 cycles.
However, supercapacitors can store only small amounts of power and don’t scale easily. The EFC mirrors a redox flow battery, however, in its ability to store utility-scale amounts of energy. By building bigger reservoirs for the slurry, it is possible to increase energy storage. “Additionally, systems based on aqueous electrolytes are safe and environmentally friendly, unlike most batteries,” Kumbur says.
No comments:
Post a Comment